
The Arabic numbering system is the most widely accepted today. Many periodic tables include both Roman and Arabic numbers. The modern IUPAC system uses Arabic numbers 1-18, simply numbering the columns of the periodic table from left to right.It is classified as a transitional metal. The CAS system used letters to differentiate main group (A) and transition (B) elements. Silver is the second element in the eleventh column of the periodic table.The older IUPAC system used Roman numerals together with letters to distinguish between the left (A) and right (B) side of the periodic table.Three systems have been used to number families and groups: It is also soft and the most malleable and ductile of the elements an ounce (31.1 grams gold is weighed in troy ounces) can be beaten out to 187 square feet (about 17 square metres) in extremely thin sheets called gold leaf. It is a good conductor of heat and electricity. Recognizing Families on the Periodic TableĬolumns of the periodic table typically mark groups or families. Gold is one of the densest of all metals. Noble Gases: - Group 18 (VIIIA) - 8 valence electrons.Halogens: - Group 17 (VIIA) - 7 valence electrons.Oxygen Group or Chalcogens: - Group 16 (VIA) - 6 valence electrons.Nitrogen Group or Pnictogens: - Group 15 (VA) - 5 valence electrons.

Carbon Group or Tetrels: - Group 14 (IVA) - 4 valence electrons.Boron Group or Earth Metals: Group 13 (IIIA) - 3 valence electrons.Transition Metals: Groups 3-12 - d and f block metals have 2 valence electrons.Silver is a transition metal in group 11, period 5, and the d-block of the periodic table. Alkaline Earth Metals: Group 2 (IIA) - 2 valence electrons Diagram of the nuclear composition, electron.Alkali Metals: Group 1 (IA) - 1 valence electron.Many chemists and chemistry textbooks recognize five main families:Īnother common method of categorization recognizes nine element families: However, there are different ways of categorizing elements into families. The transition metals are elements between Groups 2 and 13 in the periodic table.

Because element properties are largely determined by the behavior of valence electrons, families and groups may be the same. Chemists classify silver as a transition metal. Element groups, on the other hand, are collections of elements categorized according to similar properties.
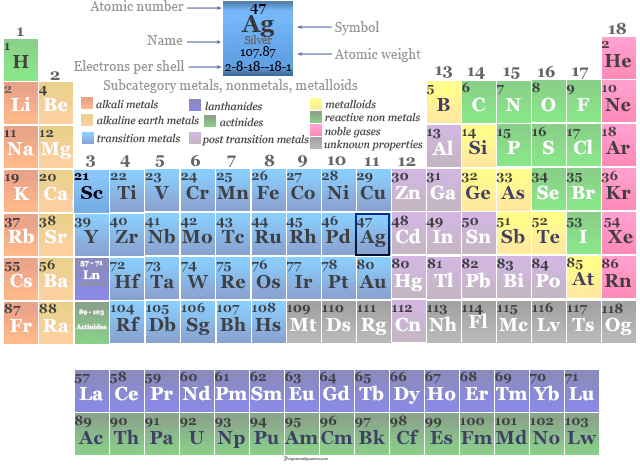
Silver Properties Based on Location This location places silver in the transition metal group.
#Silver on periodic table full#
This places it in the middle of the second full row (period) of the table. The characteristics of the elements in these families are determined primarily by the number of electrons in the outer energy shell. Silver is the 47 th element on the periodic table. Elements are classified into families because the three main categories of elements (metals, nonmetals, and semimetals) are very broad. Up to date, curated data provided by Mathematica's ElementData function from Wolfram Research, Inc.Element families are indicated by numbers located at the top of the periodic table.Īn element family is a set of elements sharing common properties. Specific Heat: Value given for solid phase. Technical data for the element Silver in the Periodic Table H


 0 kommentar(er)
0 kommentar(er)
